Catalysts
Catalysts in General
In the simplest definition, a catalyst is a substance which speeds up or causes a chemical reaction, but remains unchanged. There are two general types of catalytic systems; homogeneous and heterogeneous. Homogeneous catalysis refers to systems in which the catalyst and the reactants are in the same phase, such as liquid catalyst acting upon liquid reactants. Heterogeneous systems are more common to air pollution control. These systems operate with the catalyst and the reactants in different phases. A good example of this is the catalytic converter in automobiles. In this case the catalyst is a solid material, while the reactants are present as gases in the automobile exhaust.
Some basic characteristics of catalysts are as follows
- In the catalytic reaction, the catalyst is unchanged when the reaction is complete, although at different phases of the reaction the catalyst may be present as a different chemical compound.
- When more than one possible reaction can occur between reactants, the catalyst may be designed to promote a single specific desired reaction. This phenomenon is called selectivity, and lends its name to “selective catalytic reduction” referring to the ability of SCR catalyst to catalyze primarily the nitrogen oxide reduction reaction.
- The rate of reaction is generally proportional to the amount of catalyst present. For solid catalyst, parameters such as surface area, number of active sites, and porosity are all important to reaction rate.
SCR Catalysts
Current formulations of SCR catalyst are based upon patented discoveries by the Japanese and are typically comprised of vanadium pentoxide (V2O5) as the active material deposited on or incorporated with a substrate. The V2O5 composition typically ranges between one and five weight percent depending on the specific design. Tungsten and molybdenum are often included in the catalyst formulation. The catalyst substrate is typically composed of relatively pure titanium dioxide (TiO2), although some manufacturers use modifications to this standard material.
Catalysts for use in SCR technology are generally divided into three types, honeycomb, plate, and hybrid (sometimes called corrugated), referring to the particular geometry and manufacturing process used. “Pitch” is used as a standard method of denoting the opening size of the flue gas channels that are formed via the catalyst geometry. Large-pitch catalysts are typically applied to coal-fired boilers, while small-pitch catalysts are applied to installations where no particulate (dust) is expected, such as when natural gas is the only fuel. SCR reactors are typically constructed of several layers (two to four) of catalyst. The three geometries of SCR catalysts are shown below. These are coal-fired catalysts, but gas-fired catalysts will have similar geometries, just much smaller pitches.
Plate catalysts use a metallic screen to help support the ceramic-like catalyst material, which includes the catalyst substrate and the active catalytic components. Honeycomb catalysts are typically extruded from a clay-like ceramic, forming square channels – this results in a monolith of ceramic material which does not require a screen support. Hybrid catalysts have characteristics of both plate and honeycomb catalysts, and have a corrugated appearance. In all cases, the catalyst substrate consists primarily of titanium dioxide.
Plate Type Catalyst

Honeycomb Type Catalyst

Hybrid/Corrugated Type Catalyst

Catalysts may also promote side reactions, which may be beneficial or detrimental, depending on the circumstances. In the case of SCR catalysts, one primary detrimental side reaction is the oxidation of SO2 to SO3. This reaction is undesirable because it the SO3 that is formed leads to fouling in the air preheater, and potential emissions issues. For facilities that have little or no SO2 in the flue gas, SO2 oxidation may not be a concern. An example of a beneficial side reaction is the oxidation of mercury (for coal-fired boilers). This side reaction aids in the capture of mercury, and is therefore very important for some facilities.
As a result of the many technical issues related to SCR technology, catalyst design is extremely complicated and vendor offerings for any particular installation will represent a compromise between catalyst volume and cost, deNOx activity, SO2 conversion, and pressure drop. Mercury oxidation may also be a consideration. Catalyst manufacturers typically address the many catalyst design issues by utilizing their technical expertise developed over many years of experience.
Catalysts have a very high specific surface area due to the porosity of the substrate – this is sometimes termed “BET” surface area. This internal surface area should not be confused with the geometric surface area which is a function of the physical geometry of the catalyst structure. The internal surface area can be thousands or millions of times greater than the simple geometric surface area would indicate. The high internal surface area allows for an adequate number of active sites to accomplish the degree of reaction needed. When active sites are reduced in number, or are otherwise rendered incapable of performing their function, the term “deactivation” is used. The figure below shows an elementary depiction of an SCR catalyst surface, with internal pores and active sites.
Microscopic Diagram of Catalyst
with Internal Pores and Active Sites
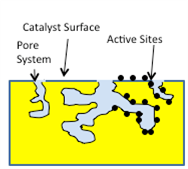
The specific design of any particular catalyst addresses many different factors. These include the total porosity, pore size distribution, internal surface area, geometrical surface area, vanadium content, promoter content (promoters are chemicals that may help increase activity or inhibit SO2 oxidation, for instance), dispersion of the vanadium and promoters, physical strength, etc. Thus, many technical factors must be addressed when designing a catalyst and the number of firms capable of providing SCR catalyst on a commercial basis is relatively small. When a particular catalyst design is offered for an application, all of these design factors must be considered to insure that an offering is being made which will meet the required guarantees.
W.S. Hinton & Assoc. is extremely well versed in the design of SCR catalysts and has close relationships with all of the major catalyst suppliers in the US. There is no one best catalyst supplier, but there is one best catalyst for your particular application. We can help you find the right solution for your needs.
