Fly Ash
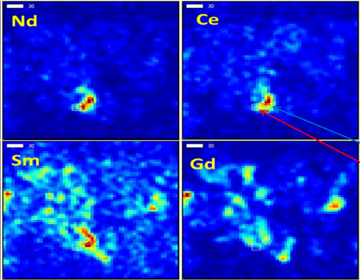
W. S. Hinton & Associates has conducted a number of research projects associated with fly ash from coal-fired power facilities. In particular, addressing the problems associated with ammoniated fly ash in both the plant setting as well as in end-use applications has been a focus (see additional information below). We have assessed numerous beneficiation processes to remove the ammonia from fly ash. Other focus areas have been fly ash toxicity and the associated regulatory concerns. Novel fly ash end-use technologies have been investigated. Other areas of interest include the recovery of metals and other beneficial products, such as rare earth elements and cenospheres.
Fly Ash Ammonia-Related Behavior
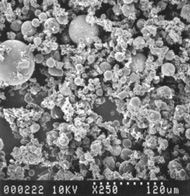
Electron Microscope Image of Fly Ash Particles – 250X Magnification
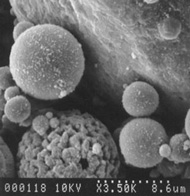
Electron Microscope Image of Fly Ash Particle – 350X Magnification
The behavior of fly ash, both in the flue gas, and in the environment, is an important issue for many coal-fired power plants. Fly ash impacts numerous chemical and physical processes, and is especially important in mercury oxidation and capture, ammonia adsorption, and SO3 adsorption. We have conducted numerous projects related to fly ash behavior, including catalytic processes, adsorption and desorption phenomenon, leaching behavior, and behavior in products such as cement and concrete, etc.
One area of special interest is the behavior of fly ash with respect to ammonia. The use of ammonia with deNOx technologies or for flue gas conditioning can have a substantial balance-of-plant impact. Ammonia tends to adsorb on fly ash within the flue gas train as both free ammonia and ammonium-sulfate compounds. This ammonia can then desorb during subsequent transport, disposal, or use of the fly ash. This desorption of ammonia presents several technical and environmental concerns.
Various fly ashes adsorb ammonia at different rates and equilibrium concentrations. For instance, high sulfur eastern bituminous coal ashes may adsorb nearly all of the available ammonia resulting in relatively high ammonia concentrations on the ash. Ashes more basic in nature with very low sulfur content tend to adsorb much less ammonia. These variations in the adsorption capabilities of the ash make predictions difficult as to the exact amount of ammonia that will be adsorbed with respect to the amount of ammonia slip that is available. Typical ammonia-on-ash concentrations range from less than 30 ppmw to several hundred ppmw for systems experiencing ammonia slip concentrations of 2 to 5 ppmv. Thus, some units operating with very low amounts of ammonia slip (<1 ppmv) may experience ammonia-on-ash concentrations of over 100 ppmw, while other units with relatively high ammonia slip may have ashes with very low levels of adsorbed ammonia (<50 ppmw). Thus, it is important that all potential problems associated with ammonia on fly ash are well understood, prior to specifying maximum ammonia slip concentrations to potential technology suppliers.
Ammonia on fly ash presents several problems with the use or disposal of the fly ash. Typically, moisture is the controlling factor in the rate and amount of ammonia that will desorb from the fly ash. In cases where ash is placed in a pond, most or all of the ammonia may desorb into the pond water, causing environmental damage if the wastewater is released directly to adjacent tributaries and rivers. Landfilling of ammoniated fly ash may also cause leachate or runoff waters to have high concentrations of ammonia, again presenting an environmental threat.
Ammonia from contaminated fly ash desorbing into the air is also a potential problem. Ammonia in air concentrations at landfills, near processing equipment, or associated with the use of ammoniated fly ash in commercial products has the potential to produce hazardous concentrations, or concentrations that are objectionable to personnel. The behavior of ammoniated fly ash associated with its use in concrete mixtures is of special concern since a great deal of ash is used for this purpose. Ashes with high amounts of ammonia may be unacceptable for use in concrete due to odor problems associated with the mixing and pouring of the concrete. These problems are very much a function of the specific mixing conditions and final use of the concrete, and are therefore often intermittent.
